Radiocarbon is integrated into carbon-bearing organisms during their lifetime. Radiocarbon is formed in the upper atmosphere through the reaction between cosmic rays and nitrogen (14N), forming three different types of carbon – one radioactive form (14C) as well as two stable forms (12C, 13C).
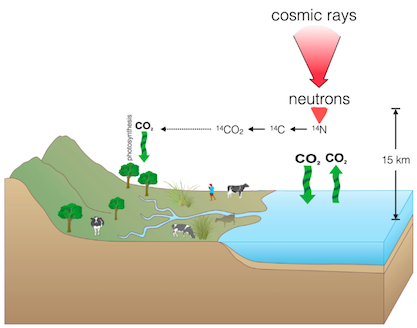
During its life, terrestrial plants and animals remain in chemical equilibrium with the atmosphere by exchanging carbon directly with the atmosphere (i.e. plants through photosynthesis) or indirectly through its diet (i.e. animals through the consumption of plants). It will therefore have the same proportion of radiocarbon as the atmosphere during its life, and ceases to acquire radiocarbon after death. It is this equilibrium that permits organisms to be dated by measuring radiocarbon within its tissues.
Marine organisms also integrate radiocarbon into their tissues, but not in equilibrium with the atmosphere – instead in equilibrium with the surrounding ocean water. Radiocarbon is transferred from the atmosphere into the ocean through mixing along the air-sea interface. This mixing is not necessarily constant and the oceans represent a large reservoir of radiocarbon, resulting in a difference between the radiocarbon value measured in terrestrial and marine organisms living at the same time.
Surfaces of oceans and other bodies of water have two sources of radiocarbon – atmospheric carbon dioxide and the deep ocean. Deep waters in oceans acquire radiocarbon from both mixing with the surface waters as well as from the radioactive decay already occurring at their levels. Studies show that equilibration of CO2 with radiocarbon in surface water is of the order of 10 years – resulting in large differences between the measured age (using a terrestrial calibration curve) and the actual age of the organism in question. The degree of equilibration of carbon dioxide in deep water remains unknown.
The level of radiocarbon within the atmosphere and ocean have not been constant through time, instead they have fluctuated due to both natural and anthropogenic processes (e.g. magnetic field variations, solar activity, cosmic ray flux and nuclear weapons testing). Due to this variability – and the delta between the atmosphere and ocean radiocarbon – individual calibration curves have been developed for each sphere. The atmospheric calibration curve (INTCAL20) was developed using terrestrial archives – mainly tree-rings – whereas the marine calibration curve (MARINE20) developed using Uranium-Thorium dated corals, speleothems and sedimentary sequences.
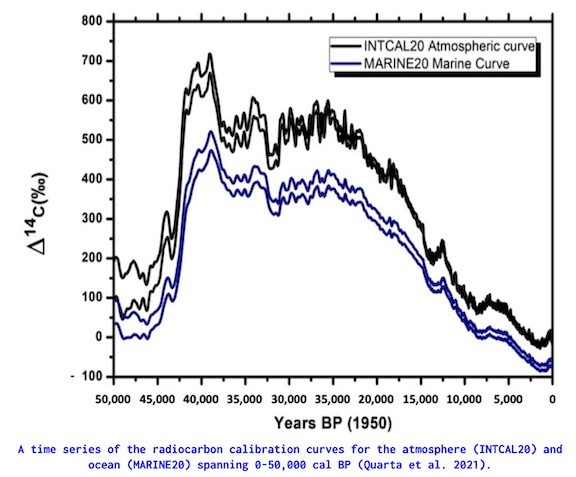
When comparing the terrestrial INTCAL20 to the ocean MARINE20, it is clear that while the curves demonstrate similar long-term trends, ocean organisms demonstrate a higher value of Δ14C compared to terrestrial organisms across the entire measured period from present (1950) to 50,000 years before present. This is known as the Marine Reservoir Effect (MRE), which has profound implications to dating marine organisms.
In order to account for this marine deviation, the local reservoir effects must be taken into account and used to calibrate the date of any marine sample. It is possible to view the available marine calibrations relevant to your sample online on the Marine20 Database and calculate the reservoir offset (ΔR) closest to your sampling site. This information should be provided when submitting your samples to Beta Analytic for radiocarbon dating.
For inquiries on radiocarbon dating costs and turnaround times, please contact us using this form.
Image References:
Radiocarbon diagram: Alves, E.Q., Macario, K., Ascough, P. and Bronk Ramsey, C., 2018. The worldwide marine radiocarbon reservoir effect: definitions, mechanisms, and prospects. Reviews of Geophysics, 56(1), pp.278-305.
Calibration figure: Quarta, G., Maruccio, L., D’Elia, M. and Calcagnile, L., 2021. Radiocarbon Dating of Marine Samples: Methodological Aspects, Applications and Case Studies. Water, 13(7), p.986.

You might also be interested in these free webinars available on demand:
Isotopes & Dating in Marine Environments (d18O, d13C, B, Pb, Sr, Nd, Hf)
Sediments – Dating & Environmental Reconstructions
U-Th Dating vs Carbon-14 Dating
This entry was posted on Monday, May 8th, 2023 and is filed under Beta Analytic Updates .